VIDEO ANSWER: In the 30 degree main, hydrogen isn’t descend with the more like negative atom, so hydrogen bonding isn’t possible in the 3 degree main. Nitrogen…
Question Video: Identifying Which Compound Could Form Hydrogen Bonds within Its Molecules | Nagwa
The next compound is not flow right. The next compound has given US CH three, CH two, the whole twice for which the next component is given us CH three, the whole twice and into … identify the compound that foes not have hydrogen bonding. A. (CH3)3 SO4 B. H20 C. CH3 CH2 OH D. HCl E. CH3 CH2 NH2 … Mr Mahendra Rathore. We don’t have your

Source Image: chemistry.stackexchange.com
Download Image
Chemistry College answered • expert verified Identify the compound that does NOT have hydrogen bonding. A) CH3NH2 B) H2O C) (CH3)3N D) CH3OH E) HF Advertisement Expert-Verified Answer question No one rated this answer yet — why not be the first? 😎 priyankatutor does not have hydrogen bonding because of the absence of electronegativity difference.

Source Image: pubs.acs.org
Download Image
Hydrogen Bond | Definition, Types & Examples – Video & Lesson Transcript | Study.com
Study with Quizlet and memorize flashcards containing terms like How many compounds, of the ones listed below, have hydrogen bonding? CH3CH2CH2NH2 CH3CH2NHCH2CH3 (CH3CH2)2NCH2CH3, Identify the compound that does not have hydrogen bonding., In a liquid, the energy required to increase the surface area by a unit amount is called.. and more.

Source Image: study.com
Download Image
Identify The Compound That Does Not Have Hydrogen Bonding
Study with Quizlet and memorize flashcards containing terms like How many compounds, of the ones listed below, have hydrogen bonding? CH3CH2CH2NH2 CH3CH2NHCH2CH3 (CH3CH2)2NCH2CH3, Identify the compound that does not have hydrogen bonding., In a liquid, the energy required to increase the surface area by a unit amount is called.. and more.
Chemistry Chemistry questions and answers Identify the compound that does NOT have hydrogen bonding. Answer A) CH3NH2 B) H2O This problem has been solved! You’ll get a detailed solution from a subject matter expert that helps you learn core concepts. See Answer Question: Identify the compound that does NOT have hydrogen bonding.
How to Identify Organic Compounds | Chemistry | Study.com
Jan 30, 2023The evidence for hydrogen bonding. Many elements form compounds with hydrogen. … Thus, we see molecules such as PH 3, which do not participate in hydrogen bonding. PH 3 exhibits a trigonal pyramidal molecular geometry like that of ammonia, but unlike NH 3 it cannot hydrogen bond. This is due to the similarity in the electronegativities of
Cooperative C−H···O Hydrogen Bonding in CO2−Lewis Base Complexes: Implications for Solvation in Supercritical CO2 | Journal of the American Chemical Society

Source Image: pubs.acs.org
Download Image
University of Ottawa NMR Facility Blog: Chemical and Magnetic Equivalence
Jan 30, 2023The evidence for hydrogen bonding. Many elements form compounds with hydrogen. … Thus, we see molecules such as PH 3, which do not participate in hydrogen bonding. PH 3 exhibits a trigonal pyramidal molecular geometry like that of ammonia, but unlike NH 3 it cannot hydrogen bond. This is due to the similarity in the electronegativities of
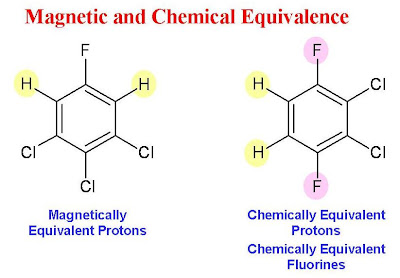
Source Image: u-of-o-nmr-facility.blogspot.com
Download Image
Question Video: Identifying Which Compound Could Form Hydrogen Bonds within Its Molecules | Nagwa
VIDEO ANSWER: In the 30 degree main, hydrogen isn’t descend with the more like negative atom, so hydrogen bonding isn’t possible in the 3 degree main. Nitrogen…
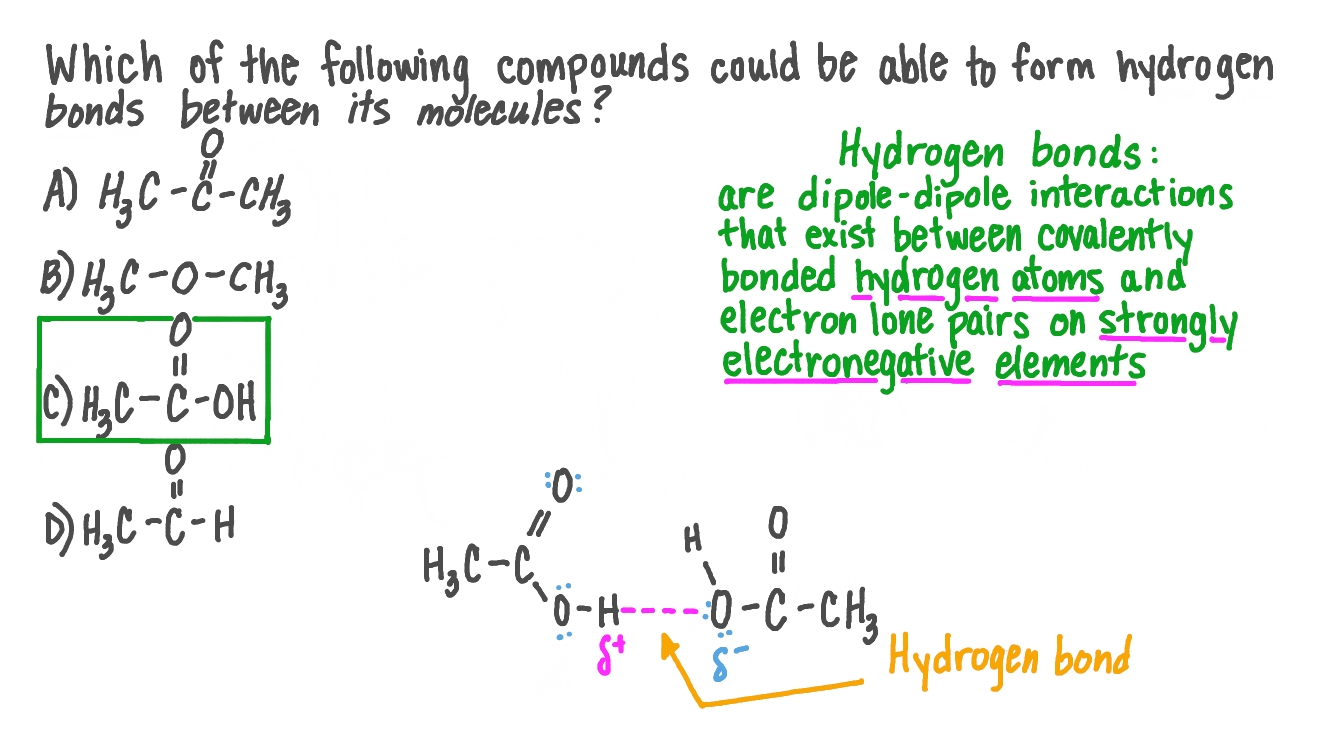
Source Image: nagwa.com
Download Image
Hydrogen Bond | Definition, Types & Examples – Video & Lesson Transcript | Study.com
Chemistry College answered • expert verified Identify the compound that does NOT have hydrogen bonding. A) CH3NH2 B) H2O C) (CH3)3N D) CH3OH E) HF Advertisement Expert-Verified Answer question No one rated this answer yet — why not be the first? 😎 priyankatutor does not have hydrogen bonding because of the absence of electronegativity difference.

Source Image: study.com
Download Image
Describing the Properties of Ionic Solids | Chemistry | Study.com
VIDEO ANSWER: In the 30 degree main, hydrogen is not descend with the more like negative atom, so it is not possible to have hydrogen bonding in the 3 degree main. Nitrogen… Get 5 free video unlocks on our app with code GOMOBILE

Source Image: study.com
Download Image
Multiple Hydrogen-Bonding Interactions Enhance the Solubility of Starch in Natural Deep Eutectic Solvents: Molecule and Macroscopic Scale Insights | Journal of Agricultural and Food Chemistry
Study with Quizlet and memorize flashcards containing terms like How many compounds, of the ones listed below, have hydrogen bonding? CH3CH2CH2NH2 CH3CH2NHCH2CH3 (CH3CH2)2NCH2CH3, Identify the compound that does not have hydrogen bonding., In a liquid, the energy required to increase the surface area by a unit amount is called.. and more.

Source Image: pubs.acs.org
Download Image
How do you to tell when a hydrogen bond will occur?
Chemistry Chemistry questions and answers Identify the compound that does NOT have hydrogen bonding. Answer A) CH3NH2 B) H2O This problem has been solved! You’ll get a detailed solution from a subject matter expert that helps you learn core concepts. See Answer Question: Identify the compound that does NOT have hydrogen bonding.
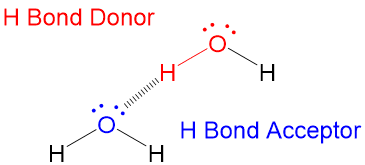
Source Image: studyorgo.com
Download Image
University of Ottawa NMR Facility Blog: Chemical and Magnetic Equivalence
How do you to tell when a hydrogen bond will occur?
The next compound is not flow right. The next compound has given US CH three, CH two, the whole twice for which the next component is given us CH three, the whole twice and into … identify the compound that foes not have hydrogen bonding. A. (CH3)3 SO4 B. H20 C. CH3 CH2 OH D. HCl E. CH3 CH2 NH2 … Mr Mahendra Rathore. We don’t have your
Hydrogen Bond | Definition, Types & Examples – Video & Lesson Transcript | Study.com Multiple Hydrogen-Bonding Interactions Enhance the Solubility of Starch in Natural Deep Eutectic Solvents: Molecule and Macroscopic Scale Insights | Journal of Agricultural and Food Chemistry
VIDEO ANSWER: In the 30 degree main, hydrogen is not descend with the more like negative atom, so it is not possible to have hydrogen bonding in the 3 degree main. Nitrogen… Get 5 free video unlocks on our app with code GOMOBILE