Expert Solution Trending now This is a popular solution! Step by step Solved in 2 steps with 2 images SEE SOLUTION Check out a sample Q&A here Knowledge Booster Learn more about Ideal and Real Gases Need a deep-dive on the concept behind this application? Look no further.
SLES 15 SP5 | Virtualization Guide
Oct 27, 2022An average balloon has a diameter of 60 feet and a volume of 1.1 × 10 5 ft 3. What is the lifting power of such a balloon? If the weight of the balloon and its rigging is 500 pounds, what is its capacity for carrying passengers and cargo? A balloon carries 40.0 gallons of liquid propane (density 0.5005 g/L).

Source Image: scribd.com
Download Image
The mathematical expression of Avogadro’s Law is: V = k × n (16.8.1) (16.8.1) V = k × n. or. V1 n1 = V2 n2 (16.8.2) (16.8.2) V 1 n 1 = V 2 n 2. where n n is the number of moles of gas and k k is a constant. Avogadro’s Law is in evidence whenever you blow up a balloon. The volume of the balloon increases as you add moles of gas to the balloon

Source Image: espncricinfo.com
Download Image
45. A balloon contains 14.0 L of air 760 torr. What will be the volume of the balloon when it is taken to a depth of 10 ft, in a swimming pool? A balloon inflated with three breaths of air has a volume of 1.7 L. At the same temperature and pressure, what is the volume of the balloon if five more same-sized breaths are added to the balloon? A weather balloon contains 8.80 moles of helium at a pressure of 0.992 atm and a temperature of 25 °C at ground level.

Source Image: bartleby.com
Download Image
A Birthday Balloon Has A Volume Of 14.1
A balloon inflated with three breaths of air has a volume of 1.7 L. At the same temperature and pressure, what is the volume of the balloon if five more same-sized breaths are added to the balloon? A weather balloon contains 8.80 moles of helium at a pressure of 0.992 atm and a temperature of 25 °C at ground level. ISBN: 9780078746376. Author: Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom. Publisher: Glencoe/McGraw-Hill School Pub Co. Solution for A birthday balloon had a volume of 14.1 L when the gas inside was at a temperature of 13.9 °C. Assuming no gas escapes, what is its volume when the….
Answered: A birthday balloon had a volume of 14.1… | bartleby
Expert Answer 100% (1 rating) Transcribed image text: Enter your answer in the provided box. Hint Solution At a birthday pool party, the temperature is 27.50°C and the atmospheric pressure is 761.4 mmHg. One of the decoration helium balloons has a volume of 6.25 L. Physics Chapter 14- Kinetic Theory of Gases | PPT

Source Image: slideshare.net
Download Image
Spring Time Theory Expert Answer 100% (1 rating) Transcribed image text: Enter your answer in the provided box. Hint Solution At a birthday pool party, the temperature is 27.50°C and the atmospheric pressure is 761.4 mmHg. One of the decoration helium balloons has a volume of 6.25 L.
Source Image: doubleslitsolution.weebly.com
Download Image
SLES 15 SP5 | Virtualization Guide Expert Solution Trending now This is a popular solution! Step by step Solved in 2 steps with 2 images SEE SOLUTION Check out a sample Q&A here Knowledge Booster Learn more about Ideal and Real Gases Need a deep-dive on the concept behind this application? Look no further.
Source Image: documentation.suse.com
Download Image
45. A balloon contains 14.0 L of air 760 torr. What will be the volume of the balloon when it is taken to a depth of 10 ft, in a swimming pool? The mathematical expression of Avogadro’s Law is: V = k × n (16.8.1) (16.8.1) V = k × n. or. V1 n1 = V2 n2 (16.8.2) (16.8.2) V 1 n 1 = V 2 n 2. where n n is the number of moles of gas and k k is a constant. Avogadro’s Law is in evidence whenever you blow up a balloon. The volume of the balloon increases as you add moles of gas to the balloon

Source Image: toppr.com
Download Image
PPT – Properties of Gases & The gas Laws PowerPoint Presentation – ID:2654009 VIDEO ANSWER: Hello too. Let’s examine the question. Given that balloon is there, this is it. The volume of the gasses filled is 2.10 and 35C. This balloon is placed in liquid nitrogen, where the temperature drops to -1, 90 60. Pressure remains

Source Image: slideserve.com
Download Image
SOLVED: Birthday balloon had a volume of 14.1 L when the gas inside was at a temperature of 13.9 °C. Assuming no gas escapes, what is its volume when the balloon warms A balloon inflated with three breaths of air has a volume of 1.7 L. At the same temperature and pressure, what is the volume of the balloon if five more same-sized breaths are added to the balloon? A weather balloon contains 8.80 moles of helium at a pressure of 0.992 atm and a temperature of 25 °C at ground level.
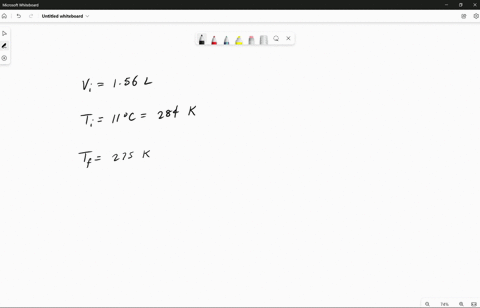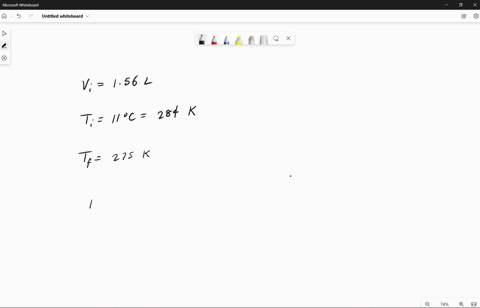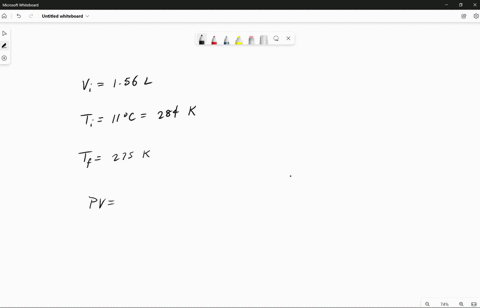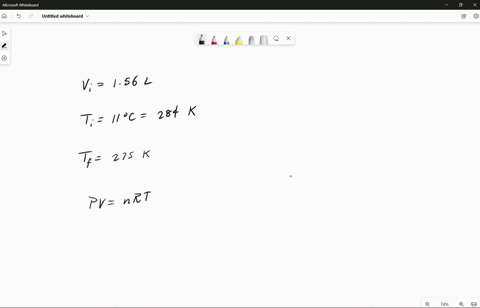
Source Image: numerade.com
Download Image
PPT – Modern Chemistry Chapter 11 GASES PowerPoint Presentation, free download – ID:5760602 ISBN: 9780078746376. Author: Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom. Publisher: Glencoe/McGraw-Hill School Pub Co. Solution for A birthday balloon had a volume of 14.1 L when the gas inside was at a temperature of 13.9 °C. Assuming no gas escapes, what is its volume when the….

Source Image: slideserve.com
Download Image
Spring Time Theory
PPT – Modern Chemistry Chapter 11 GASES PowerPoint Presentation, free download – ID:5760602 Oct 27, 2022An average balloon has a diameter of 60 feet and a volume of 1.1 × 10 5 ft 3. What is the lifting power of such a balloon? If the weight of the balloon and its rigging is 500 pounds, what is its capacity for carrying passengers and cargo? A balloon carries 40.0 gallons of liquid propane (density 0.5005 g/L).
45. A balloon contains 14.0 L of air 760 torr. What will be the volume of the balloon when it is taken to a depth of 10 ft, in a swimming pool? SOLVED: Birthday balloon had a volume of 14.1 L when the gas inside was at a temperature of 13.9 °C. Assuming no gas escapes, what is its volume when the balloon warms VIDEO ANSWER: Hello too. Let’s examine the question. Given that balloon is there, this is it. The volume of the gasses filled is 2.10 and 35C. This balloon is placed in liquid nitrogen, where the temperature drops to -1, 90 60. Pressure remains